Respiration is an essential process of life. Almost all organisms require the transportation of oxygen to all the cells for survival. Hemoglobin and myoglobin are two globular proteins which help in cellular respiration. Hemoglobin and myoglobin differ from each other based on the capability of the binding oxygen molecule with the heme proteins.
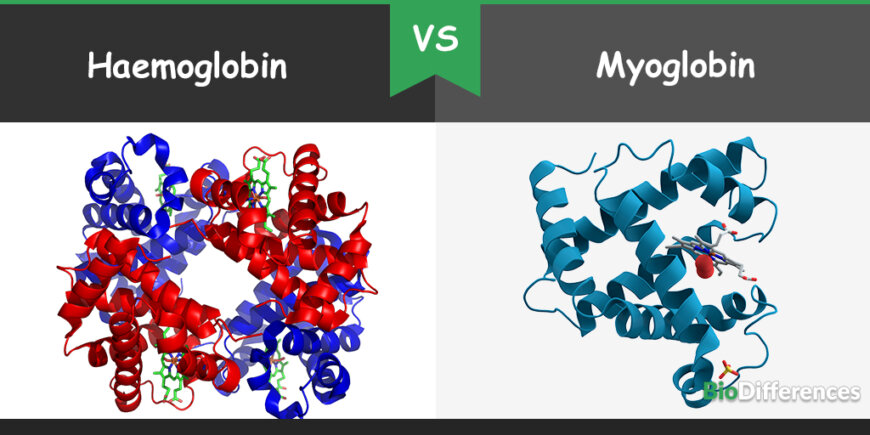
Hemoglobin is known as tetrameric hemoprotein found in erythrocytes, while myoglobin is called monomeric protein found in muscles where it plays a role as an intracellular storage site for oxygen. Hemoglobin is present systemically all over the body, whereas myoglobin is found in muscular tissues only. Hemoglobin has a low affinity for oxygen binding but presents in high concentration, whereas myoglobin has a high affinity for oxygen binding but present in low concentration in the body.
Contents
Comparison Chart
| Basis for Comparison | Hemoglobin | Myoglobin |
| Definition | Hemoglobin is a protein in RBCs that transports oxygen to the body’s organs and carbon dioxide from the body’s organs to the lungs. | Myoglobin is an iron and oxygen-binding protein found in the muscles of vertebrates and mammals. |
| Number of Chains | It contains four chains | It contains a single polypeptide chain |
| Type of Structure | A tetramer | A monomer |
| Level of structure | Exhibits quaternary structure | Exhibits tertiary structure |
| Molecular weight | 64 kDa | 16.6 kDa |
| Binds | Binds to carbon dioxide, carbon monoxide, oxygen, hydrogen ions and NO. | Binds to oxygen tightly and firmly |
| Location | Systemically in all over the body | In muscles |
| Types of curves | Sigmoid binding curves | Hyperbolic curves |
| Abbreviation | Hb | Mb |
| Affinity for oxygen binding | Low affinity | High affinity |
| Function | It carries oxygen to the whole body with blood | Myoglobin supplies oxygen to the muscles which are used in starvation. |
| Concentration in Blood | High concentration in the blood | Low concentration in the blood |
What is Hemoglobin?
Hemoglobin (Hb) is the heme protein found in RBCs carrying oxygen from the lungs to the tissues of the body and returns back carbon dioxide from the tissue back to the lungs. It is a well-known oxygen-carrying pigment and a vital part of sustaining life.
Hb has less affinity for binding oxygen and its concentration is higher in red blood cells (RBCs). So, when oxygen combines to the first subunit of hemoglobin, it converts into the quaternary structure of the protein and makes it easier for other molecules’ binding.
There must be a standard level of hemoglobin concentration in the body, which may range widely according to the age and sex of the person. When the level of the Hb in RBCs is low, the anemic condition starts.
Structure of Hemoglobin
Hemoglobin has a heme group, which is a protein and presents noncovalently. The difference lies in the part of globin t that has a different arrangement of amino acids in different animals. Heme is present as central iron and hook up with four pyrrole rings. The iron is in the ferric ion form, while the pyrrole rings are attached by methylene bridges.
Globin is the protein part that is a dimer of the heterodimer, which means proteins molecules are connected with two alpha, two beta, delta, gamma, or epsilon chains. Types of globulin chains depend on the type of hemoglobin. The globulin chain has a porphyrin compound throughout the body.
Hemoglobin includes two alpha and two beta subunits where each alpha subunit has 144 residues and the beta subunit has 146 residues.
Types of Hemoglobin
Hemoglobin is of following types:
- Hemoglobin A1
- Hemoglobin A2
- Hemoglobin A3
- Embryonic hemoglobin
- Glycosylated hemoglobin
- Fetal hemoglobin
Functions of Hemoglobin
It performs the following functions:
- It acts as a carrier for oxygen as well as for carbon dioxide.
- It plays a function in RBCs metabolism.
- It acts as an active physiological catabolite.
- It plays a role in maintaining pH.
- It gives the red color to the blood.
What is Myoglobin?
Myoglobin works for cellular respiration of muscle cells only, by receiving oxygen from the RBCs and carry it to mitochondrial organelles of muscle cells. As a result, this oxygen is used for cellular respiration to create energy.
Myoglobin is a type of heme protein serving as an intracellular storage site for oxygen. During the deficiency of oxygen, the bound oxygen known as oxymyoglobin is released from its bound form and is used for other metabolic purposes.
Myoglobin has a tertiary structure and easily soluble in water. Its parts that are exposed on the surface of the molecules are hydrophilic, while the molecules which are packed into the interior of the molecules are hydrophobic. Myoglobin has a molecular weight of 16,700, which is gone forth to that of hemoglobin.
Structure of myoglobin
The structure of myoglobin is similar to the structure of hemoglobin, but it contains non-helical regions from A through H, which is right-handed alpha helices and 8 in number.
Myoglobin also has a protein called heme, which has iron and gives a red and brown color to the proteins. It exists in the secondary structure of the protein, having a linear chain of amino acids. It consists of one subunit of alpha helices and beta sheets and the presence of hydrogen bond marked its stabilization.
Myoglobin helps in the transportation and storage of oxygen in muscle cells, which aids during the working of muscles by providing energy. The binding of oxygen is very tightly with myoglobin because venous blood combines more firmly than hemoglobin.
Myoglobin is usually found in muscles, which is useful for the organism during a shortage of oxygen. Whales and seals consist of a high amount of myoglobin. The efficiency of supplying oxygen is less than hemoglobin.
Functions of Myoglobin
It performs the following functions:
- Myoglobin has a strong affinity for oxygen binding, which makes it able to store it effectively in muscles.
- It provides oxygen to the body at the time of starvation and anaerobic situation.
- It carries oxygen to the muscles.
- It helps to regulate body temperature.
Key Differences
- Hemoglobin has four chains of two different types; alpha, beta, gamma and epsilon, whereas myoglobin has a single polypeptide chain.
- Hemoglobin chains form a structure of tetramer, whereas myoglobin chain is called a monomer.
- Hemoglobin binds with O2, CO2, BPH, NO and H+ whereas myoglobin binds only with O
- Hemoglobin transports oxygen along with blood systemically to the whole body, whereas myoglobin transports oxygen to the only muscles.
- Hemoglobin is abbreviated as Hb, whereas myoglobin is abbreviated as Mb.
- Hemoglobin is present in higher concentrations in red blood cells, whereas myoglobin is present in low concentrations in red blood cells.
Key Similarities
- Both have iron-containing protein as a central metal.
- Both are globular proteins.
- Both have the ligands as oxygen.
- Both give red color to the blood and muscles.
Conclusion
In conclusion, hemoglobin and myoglobin are both hemeproteins whose physiological importance is because of their ability to bind and transport of molecular oxygen. Both are different from each other in the number of chains, structures, and functions.