A solution is the homogenous mixture of solute and solvent in a liquid state. Solute and solvent are its compulsory components. A homogenous solution can be defined as a uniform state in which solute particles are dispersed evenly in solution. Solute particles are completely dissolved in solvent to form solution. Solubility is the property or ability of a solute to dissolve in solvent. Properties of the solution depend on the solute and solvent concentration or volume, surface area of solute particles and type of solvent. Properties of solution such as chemical properties (boiling point) and physical properties (color, density etc.) are studied for different purposes.
Solutions are studied and prepared in chemistry and research field for different purposes. Similarly, there are many products which are used in daily life in solution form such as soaps, medicines, tea, coffee, juices etc. There are examples of physiological solutions such as isotonic, hypertonic and hypotonic solutions in which salt are the solute and water is the solvent. These solutions are studied in medicine and pharmaceutical sciences regarding the physiology of the human body.
Contents
Comparision Chart
| Basis for Comparison | Solute | Solvent |
| Physical State | Three forms: Solid, Liquid and Gas | Mostly in liquid form, sometimes in solid or gas form |
| Solubility | Depends on solute properties (surface area etc) | Depends on solvent properties (polarity etc) |
| Boiling Point | Higher than solvent | Lower than solute |
| Example | Mixing sugar in water to form a sweet solution, sugar is solute in this solution. | Mixing sugar in water to make a sweet solution, water is solvent in this solution. |
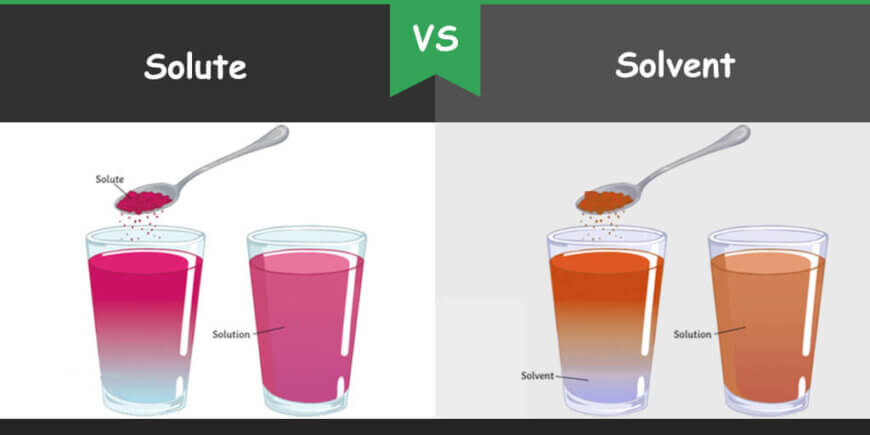
What is Solute?
A solute is a matter that is dissolved in a solution. It can be either of solid form or liquid form. But sometimes, it can also be of gaseous form. Mostly, a solute is used in solid-state. The concentration of the solution depends on the amount of the solute particles present in it with respect to the solvent. However, a solute is present in a minor concentration in its solution.
Physical and chemical properties of solutions depend on the solute. When a solute is added in the solution, properties are changed accordingly. Sometimes, a solvent may be made up of two or more solutes. When a water-soluble ionic compound or substance is mixed in water, this compound gets separated into its aqueous ions. Then this ionic compound is the solute component of that solution. When a covalent is added in a solution, it is separated into its molecules.
Solutes can be classified into polar and non-polar solutes. Polar solutes can be mixed in polar solvents whereas non-polar solutes can be mixed in non-polar solvents. Boiling points of solutes are usually higher than solvents in which they are dissolved. The solubility of solutes depends on different factors such as temperature, type of solvent used and surface area of the solute compound. The solubility of a solute can be increased in a specific solvent by enhancing the surface area. Surface area can be increased by converting into smaller particles. The solubility of gaseous solute is affected by the pressure, volume and temperature.
What is Solvent?
A solvent is a liquid in which different substances can be dissolved to form a solution. Solvent is the major component of a solution. Solvents capture the major part of the solutions and mostly of liquid form. Properties of the solvent determine the type of solutes that can be dissolved into it. Solvents can be of polar and non-polar solvent. Polar solvents are made up of polar molecules and can dissolve polar substances whereas non-polar solvents are made up of non-polar molecules. So, solvents follow a universal rule of solubility “like dissolves like”. The physical and chemical properties of a solution are usually different from the solvent found in it. Solvents can be colorful or colorless. Some solvents can be flammable and others can be inflammable. Some solvents evaporate immediately whereas other solvents have less evaporation ability. Similarly, solvents can be classified into organic and inorganic solvents. Solvents which contain carbon element in them are organic solvents where inorganic solvents do not contain carbon element. Gasoline, xylene, benzene, alcohol and acetone are some organic solvents which are widely used in chemistry experiments and industries. Water is universal solvent and commonly used in daily life. It has the ability to dissolve any type of substance such as gas, liquid or solid.
Solvents are used for different purposes and for performing different functions. These are used to regulate the temperature of solutions, either to absorb the heat produced during a chemical reaction or to increase the speed of the reaction with the solute. In organic chemistry laboratories, purification of a substance through recrystallization is a common technique. In this method, impure substance in a solvent and that solvent dissolves only substances that need to be purified. Impurities are removed through filtration. Therefore, to determine the best solvent for recrystallization is very important in this experiment.
Key Differences between Solute and Solvent
- The solute is the component of a solution which is dissolved in solvent whereas solvent is that component of solution in which solute is dissolved.
- The solute can be of all three forms such as liquid, solid and gas whereas solvent can mostly of liquid type.
- The solubility of the solute depends on specific properties of solute particles such as surface area whereas solubility of solvent depends upon properties of solvent such as polarity.
- The boiling point of the solute is higher than the solvent whereas the boiling point of the solvent is lower than solute.
Key Similarities
- Both are parts of the solution.
Conclusion
Both solute and solvent are parts of solutions which are used in everyday life and studied in chemistry, medicine and for research purpose. So the difference between solute and solvent and their properties is very significant for chemists and researchers.